PeopleWith, everyone working together to optimise the benefits of the Covid-19 Vaccination program.
Article By: Mark Bradley, CEO & Founder of PeopleWith
Article By: Mark Bradley, CEO & Founder of PeopleWith
Given the rapid development of COVID-19 vaccines and treatments, Real World Evidence (RWE) will play a critical role in understanding their safety and effectiveness. All vaccinations have a regulated process to adhere to, to ensure approval.
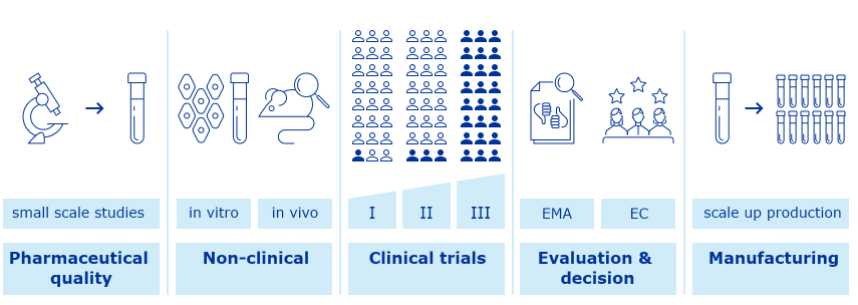
Like all medicines, COVID-19 vaccines are first tested in the laboratory (e.g. studies on their pharmaceutical quality and studies to check first the effects in laboratory tests and animals).
Then vaccines are tested in human volunteers in studies called clinical trials. These tests help confirm how the vaccines work and, importantly, to evaluate their safety and protective efficacy.
Companies first make small batches and do small scale studies to characterise and optimise the production process. They perform studies to determinate a suitable formulation that can keep vaccine components stable to the end of its shelf life.
Then the company decides whether to continue development and scale up production. To assure that the vaccine meets its intended quality profile and complies with regulatory standards, the company develops a suitable and effective quality control strategy.
Studies on pharmaceutical quality look at the individual vaccine components, the final formulation to be used and at the whole manufacturing process in detail.
The vaccine developer conducts more studies in laboratory models, using in vitro studies or animal models (in vivo studies), to show how the vaccine triggers an immune response and works to prevent infection.
Finally, the vaccine developer studies the vaccine in three phases of clinical trials, with larger numbers of volunteers in each phase.
Did you know...?
Clinical trials in human medicines, including those for COVID-19 vaccines, are authorised and managed at national level in the EU. National competent authorities and ethics committees ensure that studies are scientifically sound and conducted in an ethical manner.
Human pharmacology studies (phase I trials) generally involve between 20 and 100 healthy volunteers to confirm if the medicine behaves as expected based on laboratory tests. This can establish:
• If the vaccine triggers the expected immune response;
• If the vaccine is safe to move into larger studies;
• Which doses can be adequate.
Therapeutic exploratory studies (phase II trials) involve several hundred volunteers. The purpose of this phase is to study the best doses to use, the most common side effects and how many doses are needed.
These studies also check that the vaccine triggers a good immune response in a broader population. In certain cases, it could also provide some preliminary indications of how well the vaccine will work (efficacy).
Clinical efficacy and safety studies (phase III trials) include thousands of volunteers. This phase shows how efficacious the vaccine is at protecting against the infection compared with placebo (dummy) or alternative treatment and what are the less common side effects in those receiving the investigational vaccine.
The reduction in number of people with symptoms, severe disease or diagnosed with the infection can help to measure the efficacy of the vaccine.
This process presents a necessary minimum standard of intelligence on how the vaccine affects a selected group of patient profiles. The challenge for everyone is in understanding how it affects all of the different patients with different profiles to include people of different ages, races, ethnicity and gender. Furthermore, how does it affect people with different diagnoses, different condition severity who are being prescribed and take a range of different medications.
PeopleWith™ is the answer as it allows everyone who takes the vaccine the vehicle to capture information anonymously that allows your health profile to be understood. The PeopleWith™ app enables everyone to record symptoms as they present and progress against their profile.
The PeopleWith™ team will share consolidated intelligence on the vaccination so everyone is better informed as to the impact of the vaccination programme. Knowledge is critical at this juncture and everyone needs to work together in a structured process to ensure our experts can access this information.